Biomass chemistry is advancing to a practical stage with various conversion technologies
Updated by Taro Kamagata on September 18, 2025, 9:22 PM JST
Taro KAMAGATA
(Platinum Initiative Network, Inc.
After graduating from Keio University with a degree in economics in 1982, he joined Toppan Printing Co. In 1988, he moved to Mitsubishi Research Institute, where he was in charge of urban and regional management and public-private partnerships (PPP, PFI, etc.). He later became an executive officer and served as Director of the Regional Management Research Division, Director of the Platinum Society Research Division, and Managing Executive Officer and General Manager of the Research and Development Division, etc. He was seconded to Mitsubishi Research Institute DCS as Senior Managing Director in 2018, retired as an officer of Mitsubishi Research Institute in 2021, and became an advisor to Platinum Initiative Network in 2022. Leader of the Secretariat of the Platinum Forest Industry Initiative.
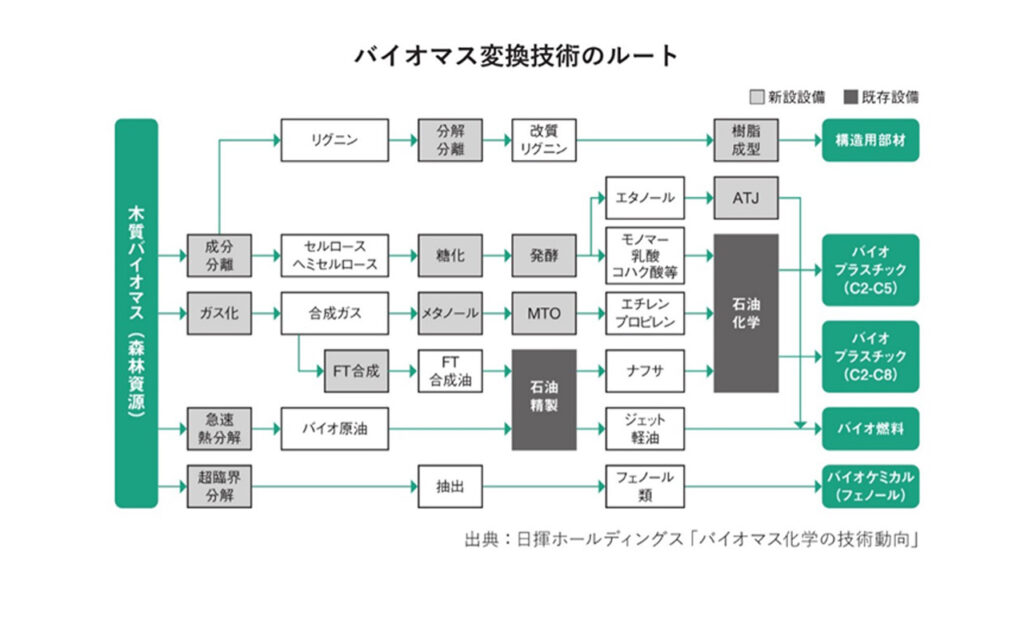
last timegave an overview of the characteristics of biomass as a resource. This issue will focus on various conversion technologies that use biomass resources to produce biochemicals and biofuels. The sites that utilize these technologies to produce products are called "biorefineries" in contrast to "petroleum refineries" that produce gasoline and kerosene from crude oil. There are two main routes for converting biomass. The other is "oilification," "gasification," and "supercritical cracking," in which wood is decomposed and converted into resources by adding heat energy, without separating it into its constituent components.
Component separation (saccharification and fermentation) is a technology to separate cellulose, hemicellulose, and lignin, which make up woody biomass, and convert cellulose and hemicellulose to fuels and chemicals through saccharification and fermentation. The following three processes are involved
(1)Pretreatment (component separation)The lignin is removed by steaming, acid treatment, alkali treatment, and explosion method, and cellulose and hemicellulose are processed into a form that can be easily saccharified.
(2)saccharificationCellulose is broken down into glucose and hemicellulose into xylose, etc. by enzymes (cellulase, etc.) and acid hydrolysis.
(3)fermentationConversion of sugars into bioethanol, lactic acid, succinic acid, etc. by yeast and bacteria.
The main uses of the products produced through the three processes are as follows
Bioethanol → Alternative fuel to gasoline
Lactic acid → Bioplastic raw materials
Succinic acid, Itaconic acid → Solvents, resins, plasticizers, etc.
This technology is characterized by its high level of maturity and ability to utilize knowledge of fermentation technology, its direct conversion to fuels and chemicals, and its significant CO2 reduction effect. On the other hand, the production cost of saccharification enzymes and high value-added utilization of lignin by-products are challenges.
Demonstrations and commercialization using agricultural residues (corn, wheat, etc.), non-edible plants, and woody biomass are progressing, mainly in Europe and the United States. However, the key to expanding commercialization lies in pretreatment costs, enzyme costs, and lignin utilization.
Lignin is one of the three major components of wood, but due to its complex structure, lignin has conventionally been treated mainly by combustion. In contrast, the "modified lignin production (acid-solvent degradation)," a Japan-originated technology, uses cedar wood as raw material and treats it with polyethylene glycol (PEG) and sulfuric acid to separate cellulose, hemicellulose, and lignin, while modifying the lignin to produce modified lignin (glycol lignin). The process is carried out by using polyethylene glycol (PEG) and sulfuric acid.
Modified lignin has thermoplasticity and is expected to be applied to high-performance materials such as resins, adhesives, coating materials, and carbon fiber reinforced resins. Cellulose and hemicellulose obtained as by-products can also be used as raw materials for bioethanol, etc., in combination with saccharification and fermentation technologies.
This is a technology to obtain bio-oil by rapidly heating wood to 400-600°C and rapidly cooling volatile components in a short period of time. Bio-oil is similar in appearance and properties to petroleum and is expected to be used as a fuel or chemical raw material.
The advantage is that wood can be processed without separation. However, the acidic and corrosive nature of the oil makes it difficult to use as-is, and reforming processes such as hydrogenation and deoxygenation are required. After reforming, the oil can be converted to gasoline or naphtha at existing oil refineries. Demonstrations and commercialization using woody biomass are underway in the United States (Ensyn), Finland (Fortum), and elsewhere. However, at this stage, the use of bio-oil is limited to heat applications (heating, steam, industrial heat, etc.), and the use of bio-oil in petroleum refineries for gasoline/naphtha production is still under consideration.
This technology decomposes wood through partial oxidation or steam reaction at 700-1000°C to produce syngas, which is mainly composed of carbon monoxide (CO) and hydrogen (H2).
Syngas obtained from gasification can be used in a wide range of applications, including the following generated
interpoint (interword separation)fuelPower generation by gas turbine or gas engine
interpoint (interword separation)chemicals: Liquid fuels (SAF, diesel), methanol, ammonia by Fischer-Tropsch synthesis
interpoint (interword separation)hydrogen: Increased hydrogen by aqueous gas shift reaction → Fuel cells and hydrogen utilization
Gasification can treat a wide variety of resources, including woody materials, agricultural residues, and waste, and can be used for a wide range of applications. However, high-cost high-temperature, high-pressure equipment, gas purification equipment, tar control measures, and stable operation are challenges to commercialization and expansion.
This technology utilizes "supercritical water," which is obtained when water is pressurized to 374°C or higher and 22.1 MPa or higher. Utilizing its high solubility and reactivity, this technology produces fuels and chemical raw materials from woody biomass.
interpoint (interword separation)Supercritical water gasification(SCWG): Converting biomass to hydrogen, methane, CO2, etc. Promising for hydrogen production.
interpoint (interword separation)Supercritical hydrothermal liquefaction(SCWL): Produces bio-crude and can be integrated with oil refineries.
interpoint (interword separation)Biomass decompositionPossible to break down cellulose, hemicellulose, and lignin → Generates chemical raw materials such as sugars, phenols, and organic acids.
The advantages of this technology are that it does not require a catalyst, has a low environmental impact, is capable of high-speed reactions, and can be applied to wet biomass, lignin, and other difficult-to-decompose resources. However, the construction and operation costs of high-temperature, high-pressure equipment are significant, and the technology is currently only in the research and demonstration stage.
In order to decarbonize the chemical industry, a shift is underway from fossil resource-dependent manufacturing processes to sustainable processes that focus on recycled and biomass feedstocks.
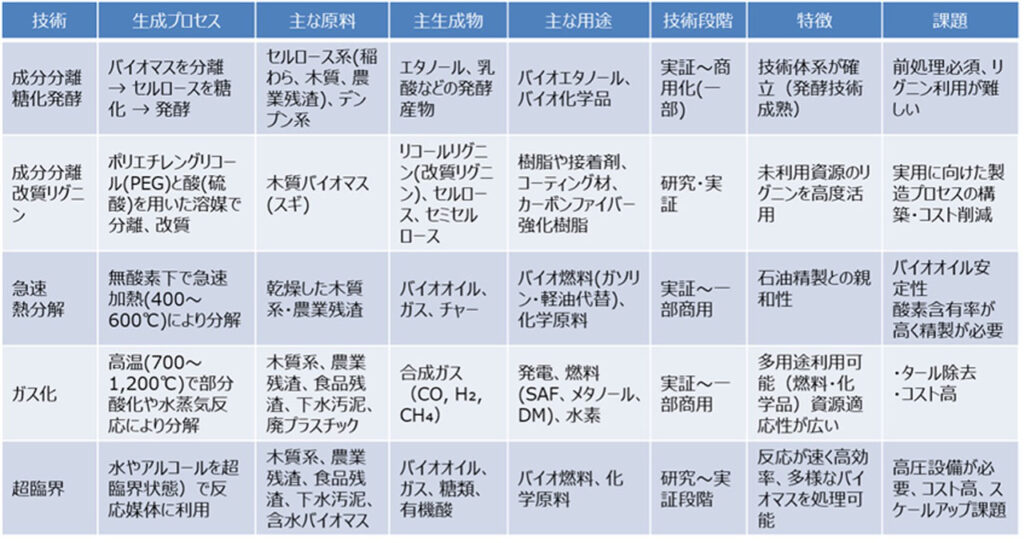
Biomass conversion technologies are already in the stages of research → demonstration → partial commercialization, and each technology has different characteristics, application conditions, and products. In the future, it is expected that they will be used selectively according to the characteristics of the feedstock, end-use applications, and potential utilization of existing facilities.
In the next issue, we will introduce case studies of companies that are introducing these technologies. (Taro Kamagata, Advisor, Platinum Initiative Network)